CENTERS OF EXCELLENCE (CoEs) FOR REGULATORY SCIENCE
The RHSC vision for the Centers of Excellence for Regulatory Science (CoEs) is to implement a sustainable platform for promoting regulatory convergence, capacity building and cooperation in PWAs relevant to medical product regulation.
The objectives of the CoEs are:
- To build human capacity in regulatory sciences to bring safe, effective, and quality medical products to patients and people as quickly as possible;
- To promote dialogue with a view towards sharing understanding in science and best practices;
- To achieve a model of sustainable operation that includes periodic updates to maintain regulatory relevancy of materials and ensures continued value to all participating entities; and
- To avoid duplication of efforts and leverage work that already exists and has a level of convergence
This is achieved through the provision of high quality training programs through partnerships with academia, regulators and industry. Curriculum and training programmes are developed by the CoE with guidance from the PWA CoE Program Committee and in line with the PWA roadmap. The RHSC provides overall oversight and periodic assessments necessary to ensure that performance of the CoEs are in line with APEC objectives.
The full list of CoEs and the respective contacts can be found here.
HOSTING INSTITUTIONS FOR A COE
CoEs are hosted and managed by universities, colleges, research centers, regulatory authorities or educational organizations that supply faculty, staff and material support.
If your institution is interested in becoming a CoE, please approach the CoE Coalition Co-Chair, Dr Jared Auclair.
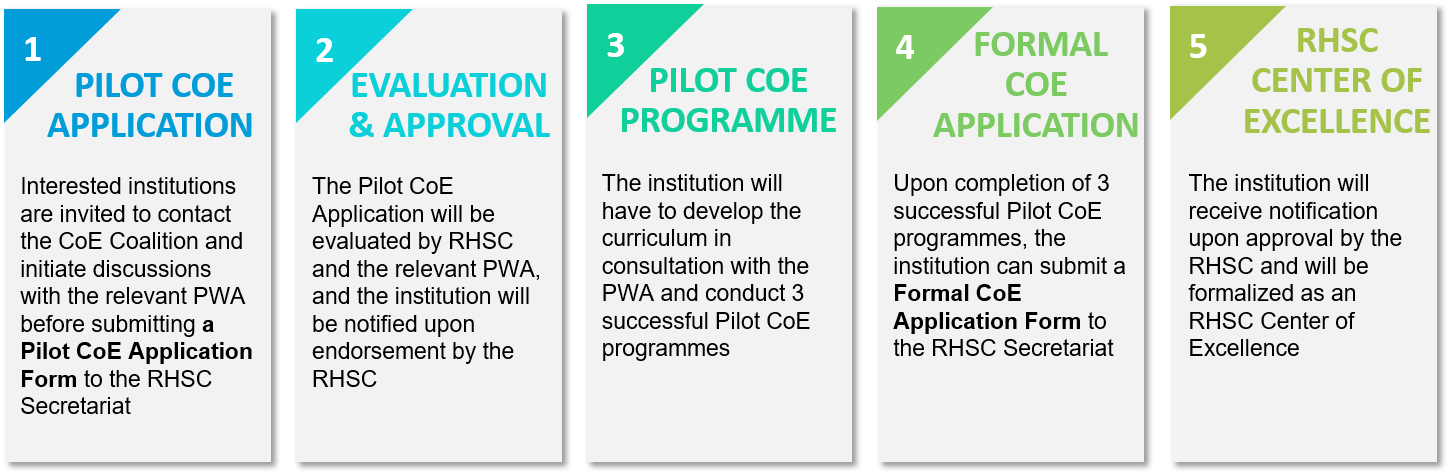
STEPS TO BECOMING A COE
SELECTION CONSIDERATIONS
- Trusted global educational/regulatory/science-setting organization and brand
- Ability to develop and deliver a training program with priorities set by the APEC RHSC
- Willingness to provide a full or part-time Director and appropriate staff to manage the CoE
- Ability & commitment to achieve objectives as agreed herein
- Ability to fund the administrative overhead over the life of the agreement (minimum 5 years)
- Demonstrated credibility in the topic area
- Location that provides, or the ability to travel to, a site easily accessed by participants
- Ability to provide qualified faculty; this could be visiting regulatory staff or other experts as required by the training program
- Ability to receive funding to support specific aspects of CoE training (e.g., to fund student travel)